News
How to properly dilute antifreeze for a car and why it is critically important
To keep your car's engine from overheating in summer and freezing in winter, you need to regularly add antifreeze to the system. This fluid helps regulate engine temperature, but it also needs to be diluted, as it is sold as a concentrate.
OBOZREVATEL figured out why antifreeze needs to be diluted and how to properly prepare a solution of the required concentration. In its undiluted form, this brightly colored liquid resists high temperatures, so it can be used in this way during the summer heat. Temperatures around 0 Celsius and below require a different approach to the use of antifreeze.
Why does antifreeze need to be diluted?
Concentrated antifreeze is a common ethylene glycol, a simple two-atom alcohol with an oily consistency. Its boiling point is +196 degrees Celsius, while it freezes at -13 degrees. Therefore, it can be used in its pure form for winter operation of a car only in regions where winters are quite mild. If one night the thermometer where you live shows -15, in the morning you risk finding your antifreeze in a frozen state, which paralyzes the entire cooling system. This is where dilution comes in. It can reduce the freezing point of the liquid to -40 or even -60 degrees.
How to dilute antifreeze?
Ordinary water is used for dilution. But it is better not to use the water that flows from the tap at home. As a rule, it is quite hard, meaning it contains a lot of salts (chlorine, calcium, magnesium, etc.) and other impurities. They can precipitate or form scale when the engine is running, which is quite risky because such an additional layer on the walls of pipes and radiators impairs heat dissipation.
How can you tell if the water is hard where you live? Just look to see if it leaves deposits and white spots on taps, plumbing, and tiles. If they do, this water is not suitable for diluting antifreeze. Therefore, it is better not to take any risks and use distilled water right away.
How to dilute antifreeze correctly?
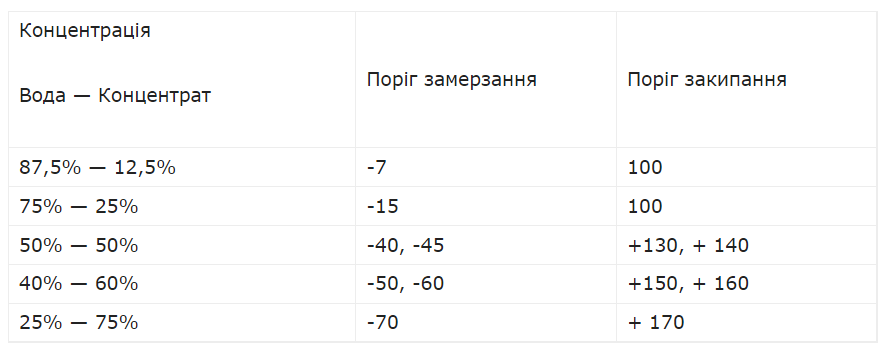
The instructions for diluting antifreeze are usually found on the canisters with this liquid. However, professional mechanics advise that you still pay attention to the minimum temperatures common in your climate zone. In Ukraine, they rarely go beyond -25 degrees. To prepare antifreeze for such conditions, you need to mix it with water in a 2:3 ratio. That is, take 3 liters of water for 2 liters of concentrate. Or 1.5 liters of water per liter of concentrate.
In this case, not only the freezing point of the antifreeze will decrease, but also the boiling point. It will drop to about +140 degrees, which is usually quite enough.
If you want to be on the safe side and prepare an antifreeze that would work at temperatures down to -45 degrees Celsius, then dilute the concentrate with water in a 1:1 ratio. Under such conditions, the boiling point will be up to +160 degrees.
In general, the table of antifreeze dilution proportions for different temperature conditions looks like this.
Only verified information is available on the Obozrevatel Telegram channel and Viber. Don't fall for fakes!